
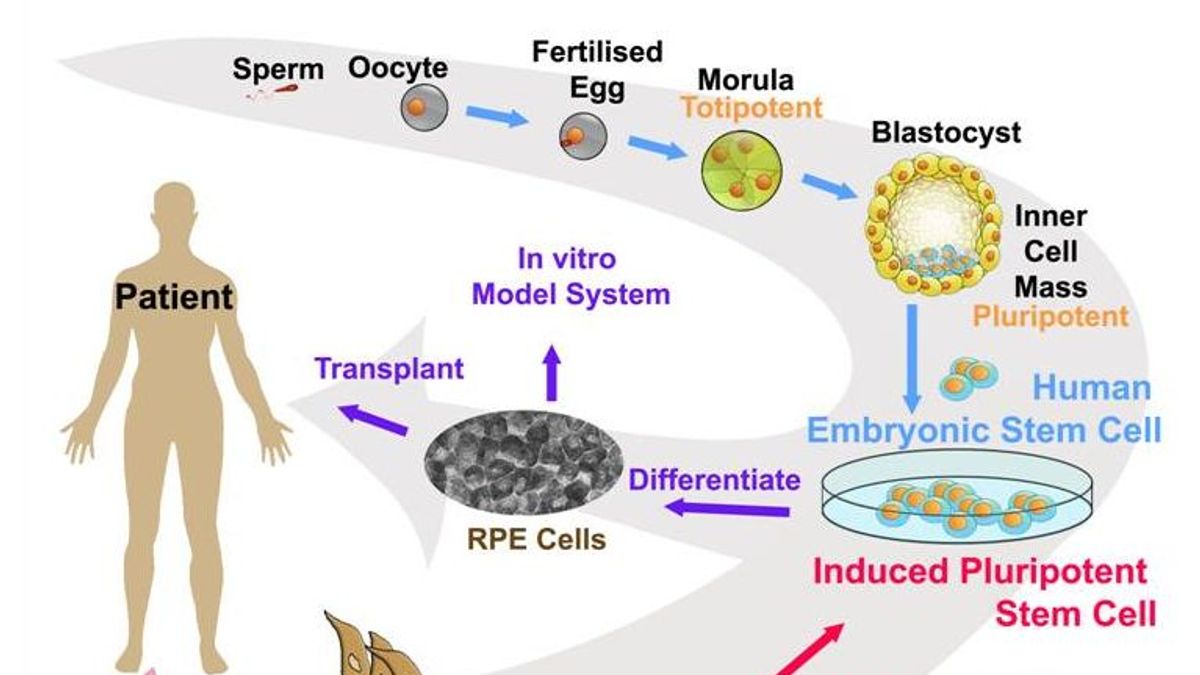
The basic paradigm in the use of PSCs for cell therapy purposes is that they are first differentiated into the desired cell types of interest, and the resulting specialized tissue-specific cells are then transplanted as cell suspensions or more complex tissue constructs into patients. In many other countries, however, the use of ESCs and/or ESC-derived cells is restricted or completely prohibited. Of note, the use of pre-implantation embryos for ESC derivation is not ethically challenging or legally banned in some countries including Canada, Sweden, Spain, France, Great Britain, Japan, Australia, Iran, and China, either because pre-implantation embryos are not considered to be “functional” human beings or their legislative bodies permit the creation or use of human embryos for research and therapeutic purposes. However, two important challenges have confined their broad application: ethical limitations, since human embryos are destroyed to produce ESCs, and immunological rejection of the cells differentiated from ESCs upon allogeneic cell transplantation. The resulting PSCs, known as ESCs, opened new avenues for research and perspectives for clinical practice. Prior to 2007, PSCs from humans could only be derived from pre-implantation embryos such as morula- or blastocyst-stage embryos. Because of these features, iPSCs have numerous biomedical applications in basic research, drug screening, toxicological studies, disease modeling, and cell therapy.
#INDUCED PLURIPOTENT STEM CELLS FULL#
Since the full pluripotency of iPSCs has been demonstrated by several studies through the most stringent test of pluripotency, i.e., tetraploid complementation (see Glossary), it is possible to derive truly pluripotent iPSCs from somatic cells. However, according to the strictest definition, genuine or bona fide iPSCs could develop into an entire embryo in conjunction with extraembryonic membranes. Like embryonic stem cells (ESCs), they can typically proliferate and self-renew indefinitely in vitro and differentiate into derivatives of all three primary germ layers (i.e., ectoderm, mesoderm, and endoderm) as well as germ cells that give rise to the gametes.

Induced pluripotent stem cells (iPSCs) are artificial stem cells produced from somatic cells through co-expression of defined pluripotency-associated factors.
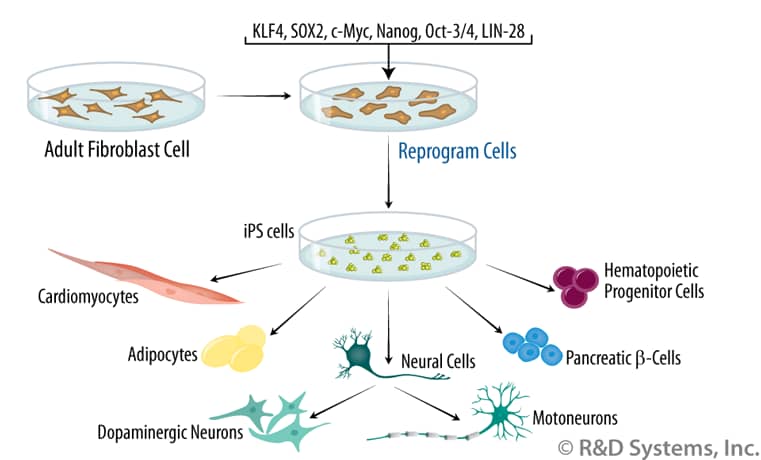
Furthermore, we present key questions and suggestions for stem cell scientists, legal authorities, and social activists investigating and working in this field. In this review, we discuss bioethical, legal, and societal concerns associated with research and therapy using iPSCs. However, various legal and ethical barriers arise when aiming to exploit the full potential of iPSCs to minimize abuse or unauthorized utilization. In addition, reprogramming technology has provided a powerful tool to study mechanisms of cell fate decisions and to model human diseases, thereby substantially potentiating the possibility to (i) discover new drugs in screening formats and (ii) treat life-threatening diseases through cell therapy-based strategies. Since they can be generated from any healthy person or patient, iPSCs are considered as a valuable resource for regenerative medicine to replace diseased or damaged tissues. iPSCs do not exist naturally and are instead generated (“induced” or “reprogrammed”) in culture from somatic cells through ectopic co-expression of defined pluripotency factors. Induced pluripotent stem cells (iPSCs) can self-renew indefinitely in culture and differentiate into all specialized cell types including gametes.


 0 kommentar(er)
0 kommentar(er)
